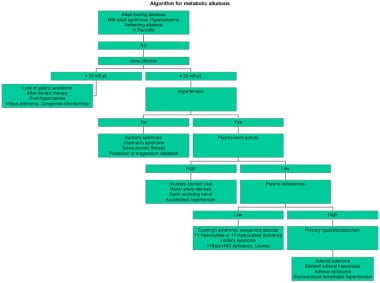
Hypochloremic alkalosis results from either low chloride intake or excessive chloride wasting. Whereas low chloride intake is very uncommon, excessive chloride wasting often occurs in hospitalized children, usually as a result of diuretic therapy or nasogastric tube suctioning. What does metabolic alkalosis mean?
Full Answer
Why is PaCO2 elevated?
An elevated PaCO2 is often present as a result of compensatory alveolar hypoventilation.
What causes HCO3- to change?
Primary changes in plasma HCO3- due to metabolic or renal factors cause compensatory changes in the ventilation which blunt the changes in the pH. 80-90% of HCO3- produced daily in the body is reabsorbed in the proximal tubule.
What is the difference between HCO3 and PCO2?
Where HCO3- represents in the plasma bicarbonate concentration and pCO2 is the plasma carbon dioxide tension in the blood. At normal conditions in the body, the CO2 production and excretion are equal and pCO2 is maintained at 40 mm Hg.
What causes bicarbonate levels to be elevated?
However, elevated urinary bicarbonate levels may also occur due to renal tubular acidosis which must be remembered in the differential diagnosis. Renal tubular acidosis (RTA) is characterized by the development of metabolic acidosis due to a defect in the ability of the renal tubules to perform normal functions [1, 6]. All forms of RTA are characterized by a normal anion gap (hyperchloremic) metabolic acidosis. This form of metabolic acidosis usually results from either the net retention of hydrogen chloride or its equivalent (such as ammonium chloride) or the net loss of sodium bicarbonate or its equivalent. The major cause of a normal anion gap acidosis in patients without renal failure is diarrhea.
How is bicarbonate reabsorption maintained?
At the cellular level, the balance between the excretion and retention of the bicarbonate in the plasma is maintained by the type A and B cells in the collecting tubules [4, 5]. Bicarbonate reabsorption in the medullary collecting tubule and in type A intercalated cell in the cortical collecting tubule is mediated by hydrogen secretion via H-ATPase pumps and passive cosecretion of chloride in the luminal membrane. Water within the cell dissociates into H+ and OH- ions. H+ ions are secreted into the lumen by H-ATPase pumps in the luminal membrane, where they primarily combine with NH3 to from NH4+. The OH- ions in the cell combine with CO2 to form HCO3- in a reaction catalyzed by carbonic anhydrase. Bicarbonate is then returned to the systemic circulation via Cl-HCO3 exchangers in the basolateral membrane. The favorable inward concentration gradient for Cl- provides the energy for HCO3- reabsorption. The intracellular bicarbonate is returned to the systemic circulation through the Cl/HCO3 exchangers in the basolateral membrane. A decline in the tubular fluid chloride concentration will promote both chloride and hydrogen secretion. H-K-ATPase pumps, which lead to both H+ secretion and K+ reabsorption, are also present in the luminal membrane. The number of these pumps increases with K+ depletion, suggesting that their main function may be to promote K+ conservation.
How long does it take for NaHCO3 to reach maximum?
The ability to enhance bicarbonate reabsorption takes 3 to 4 days to reach its maximum. Thus, there is increased NaHCO3 delivery to the collecting tubules.
Where is bicarbonate secreted in the cortical collecting tubule?
The type B intercalated cells in the cortical collecting tubule are able to directly secrete bicarbonate by reversing the location of the transporters as seen during the recovery phase of metabolic alkalosis [5]. The Cl/HCO3 exchangers are now located in the luminal membrane, leading to bicarbonate secretion into the tubular lumen. The activity of these cells is appropriately enhanced by alkalemia in an attempt to excrete the excess bicarbonate. These two mechanisms play a role in the conservation and excretion of the bicarbonate balance in the body depending on the body pH levels.
What is metabolic alkalosis?
Metabolic alkalosis is defined as a disease state where the body’s pH is elevated to greater than 7.45 secondary to some metabolic process. Before going into details about pathology and this disease process, some background information about the physiological pH buffering process is important. The primary pH buffer system in ...
What is chloride responsive metabolic alkalosis?
In chloride responsive metabolic alkalosis, this includes repletion of electrolytes, specifically chloride and potassium along with the replenishment of fluid. In scenarios, such as congestive heart failure (CHF) or edematous states, diuresis is essential using potassium-sparing diuretics.
What happens if the expected pCO2 does not match the measured value?
If the expected pCO2 does not match the measured value, an underlying metabolic alkalosis is a likely present.
What causes bicarbonate to increase in blood?
Several etiologies lead to increases in bicarbonate within the blood. The simplest of which is an overdose of exogenous sodium bicarbonate in a medical setting. Milk-alkali syndrome is a pathology where the patient consumes excessive quantities of oral calcium antacids, which leads to hypercalcemia and varying degrees of renal failure. Additionally, since antacids are neutralizing agents, they add alkaline substances to the body while reducing acid levels thus increasing pH. A pathology that is in line with normal physiology is the body’s natural compensation mechanism for hypercarbia. When a patient hypoventilates, CO2 retention occurs in the lungs and subsequently reduces pH. Over time, the renal system compensates by retaining bicarbonate to balance pH. This is a slower process. Once the hypoventilation is corrected, such as with a ventilator-assisted respiratory failure patient CO2 levels will quickly decrease, but bicarbonate levels will lag in reducing. This causes post-hypercapnia metabolic alkalosis, which is self-correcting. It is possible to calculate the expected pCO2 in the setting of metabolic alkalosis to determine if it is a compensatory increase in bicarbonate, or if there is an underlying pathology driving alkalosis using the following equation:
What is the term for a disease where the body's pH is elevated to greater than 7.45?
A decrease in pH below this range is acidosis, an increase over this range is alkalosis. Metabolic alkalosis is defined as a disease state where the body’s pH is elevated to greater than 7.45 secondary to some metabolic process.
What is the normal pH of the human body?
Normal human physiological pH is 7.35 to 7.45. A decrease in pH below this range is acidosis, an increase over this range is alkalosis. Metabolic alkalosis is defined as a disease state where the body’s pH is elevated to greater than 7.45 secondary to some metabolic process.
Which two substances regulate cerebral blood flow in the setting of acute experimental metabolic alkalosis?
Arterial carbon dioxide and bicarbonate rather than pH regulate cerebral blood flow in the setting of acute experimental metabolic alkalosis.
Popular Posts:
- 1. what happens to the proceeds when a private golf course is sold?
- 2. how to see the attendance record for a course in starfish
- 3. which of the following is a suggested guideline for using expert power? course hero
- 4. how to get rid of blurred sections on course hero
- 5. if you look carefully, you might find it for yourselves in the course of what i am going to say
- 6. what do college course numbers say about the course level collin college
- 7. why did the court find one statue invalid while the other was valid? course hero
- 8. how long is the nsam self studt cpt course
- 9. how o make nursing online course audible
- 10. how many pages of reading is considered a lot for a college course