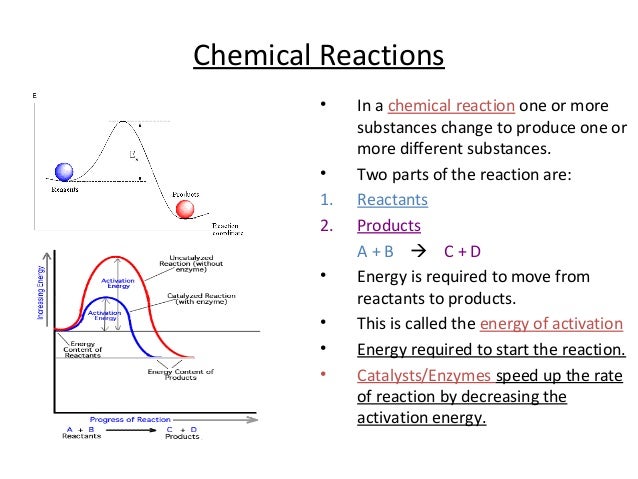
Enzymes speed up chemical reactions by lowering the amount of activation energy needed for the reaction to happen. The reactant (s) of a reaction being catalyzed by an enzyme.
Full Answer
How do enzymes accelerate chemical reactions?
Explanation: Enzymes help speed up chemical reactions in the human body. They bind to molecules and alter them in specific ways. They are the “gnomes” inside each one of us that take molecules like nucleotides and align them together to create DNA, or …
What makes enzymes speed up the chemical rate?
ENZYMES Proteins that speed up chemical reactions Activation Energy The energy needed to start a reaction in the body High ACTIVATION ENERGY means the reaction takes slower One way to speed up a reaction is HEAT, but too much heat can denature proteins, so there has to be a safer way to speed up reactions…
Does enzyme slow down any chemical reaction?
Enzymes speed up chemical reactions without being used up. F. F. 9. One enzyme can be used for many different types of chemical reactions. T. T. Choose the correct effect: write your answer in the text box A. increase B. decrease C. not change. A. increase B. decrease C. not change.
Do enzymes slow down the rate of chemical reactions?
The speed of a reaction depends on the activation energy, or the amount of energy required for the reaction to initiate. In enzyme-catalyzed reactions, the reactant is the substrate molecule. When the enzyme-substrate complex forms, the activation energy of the reaction decreases. This can happen in a number of ways. Chemical bonds in the substrate are strained by the induced …
How do enzymes help speed up chemical reactions?
Enzymes in our bodies are catalysts that speed up reactions by helping to lower the activation energy needed to start a reaction. Each enzyme molecule has a special place called the active site where another molecule, called the substrate, fits.
How do enzymes speed up chemical reactions Quizizz?
Enzyme decrease the activation energy of the reaction. Enzymes reverse the direction of the reaction. Enzymes increase the energy of the reactants.
What is a substance that speeds up the rate of a chemical reaction?
catalystA catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself being consumed during the reaction. Catalysis is the process of adding a catalyst to facilitate a reaction.
How does an enzyme's shape relate to its function?
Enzymes are specific because different enzymes have different shaped active sites. The shape of an enzyme's active site is complementary to the shape of its specific substrate or substrates. This means they can fit together.
Enzyme Structure
Enzymes are proteins that work as catalysts, or molecules that speed up biochemical reactions.
How Enzymes Affect Activation Energy
Enzymes lower the activation energy needed to begin a specific chemical reaction.
Active Sites
The shape and surface charges of an enzyme play a critical role in effecting enzymatic reactions by creating active sites where the substrate binds and the reaction occurs.
Controlling Enzyme Activity
Cells closely regulate enzyme activity through feedback inhibition, allosteric regulation, and competitive and noncompetitive inhibition.
Popular Posts:
- 1. what is the significance of absolute zero? course hero
- 2. what is the major objective of your building design course hero
- 3. how download strava course
- 4. which of the following is a business benefit from corporate social responsibility course hero
- 5. how much is the kindle kash course
- 6. what are the differences between state and federal courts? course hero
- 7. what is the age requirement fir boating education course in georgia
- 8. what is another name for golf course?
- 9. berkeley where to view course enrollment restrictions
- 10. how many yards away is the target in the california ccw course