Most researchers looking for a general training should take IRB Social & Behavioral Research. CITI certification for human subjects modules is valid for five years. See course details and first-time login instructions by clicking a course name in the table below.
Full Answer
Should I take Citi training before submitting my IRB protocol?
IRB Administration provides role-specific, peer-reviewed training written by IRB administration experts. Along with CITI Program's advantages, including our experience, customization options, cost effectiveness, and focus on organizational and learner needs, this makes it an excellent choice for IRB Administration training.
How do I complete the IRB required course?
For questions regarding IRB training, please contact the IRB Compliance Administrator in the Office of Sponsored Projects and Research Compliance at [email protected] or 713-348-3586. If you are having difficulty with the CITI training course, technical support is available at [email protected] or 305-243-7970 (8am-5pm Eastern).
Is the Citi account required by the Emory IRB?
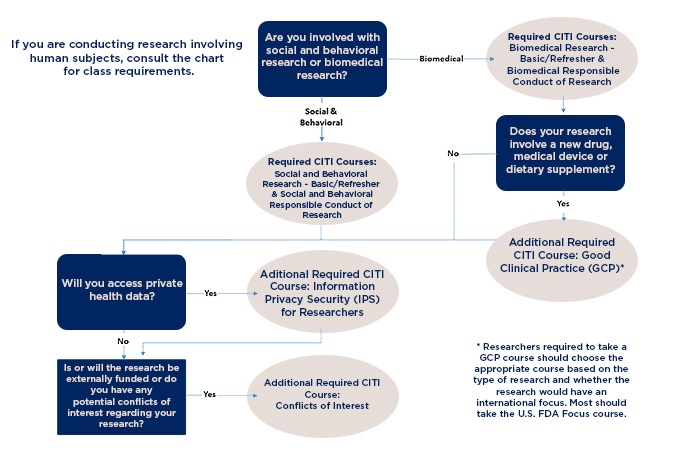
Which CITI course do I have to take? Each institution tailors the CITI program to meet their own needs and expectations, so Emory personnel must take the Emory version of CITI (or the VA version, if a VA researcher). The specific course will depend on your study area and activities:
Why choose IRB Administration Training?
The CITI Course does not need to be completed all at once, although you do have the option of doing so. Once you sign out, all the quizzes you completed will be saved, and the next time you sign onto CITI Training website, and then once again click on Basic Course, you would be able to choose the next course you need to take. Remember, you need ...
What Citi training is required for IRB?
CITI human subjects protection training is only mandatory for KSP. However, we recommend that anyone involved with human subjects complete CITI training or similar human subjects protection training. IRB members, HRPP staff and Institutional Officials also must complete CITI training.Jan 6, 2022
Is Citi training required for research?
Who must complete the Human Research Protections training? Individuals required to complete UCI Human Research training in order to engage in human subjects research and be listed on a UCI protocol, must complete the online Collaborative Institutional Training Initiative (CITI) program.
Which Citi training do I need?
Researchers should select the track that corresponds to the type of research typically conducted. Generally, the Biomedical Research Module would be required for medical, physiological or pharmacological studies.
How do I get an IRB certification?
Apply for IRB ReviewStep 1: Determine if your project requires IRB approval. ... Step 2: Complete the Mandatory Online Certification for Researchers. ... Step 3: Complete the IRB Research Project Application. ... Step 4: Prepare the Informed Consent Document(s) ... Step 5: Submit Proposal Form.More items...•Nov 14, 2018
How long does Citi IRB training take?
The time required to complete each of the basic modules varies between 10 to 30 minutes. The total time is estimated to be 2-3 hours. The course does not have to be completed in one sitting and you can enter the course at any time.
How long does it take to get IRB certified?
How long does it take to get IRB approval?Median Time (days)Range (days)IRB Review to Approval Time (total)3110 – 127Time on IRB side239 – 43Time on PI side (e.g. PI responding to contingencies)51 – 99Jun 11, 2014
Can you cheat on Citi training?
Not sharing course-related quiz questions or answers on any website, via email, photocopying, or any other means. Such sharing is considered cheating, regardless of intent. Not misrepresenting your identity or the identity of any other learner with respect to quiz completions or other learning activities.Mar 1, 2021
What is IRB training?
Human participant research ethics (IRB) training is mandatory for all personnel listed on expedited and full board protocols, and must be renewed every 5 years. For NIH-funded clinical trials, research personnel must also complete Good Clinical Practice (GCP) training.
Should I put Citi training on resume?
A CITI certification should be included in the certifications section on a resume. Include the date you became certified and do not include expired certifications. For those who specifically work in the research industry, this information may be placed in the summary section.Aug 28, 2021
What is IRB Citi?
The Collaborative IRB Training Initiative Program (CITI) is a leading online training program maintained by the University of Miami. It offers curricula in human subjects research, animal research, and the responsible conduct of research.
Do IRB members get paid?
Affiliated IRB Committee members do not receive any direct monetary compensation for participation on the board. Unaffiliated IRB Committee members will be reimbursed at an amount not to exceed $60 per month to pay for internet access and parking. Reimbursement payments will be issued quarterly.
Is Citi training free?
Learn how to take courses required by your organization. You can register now to take CITI Program content assigned to you by your organization for no charge. If you need training not provided by your organization, you can register as an independent learner and purchase the content required.
What is CITI training?
Principal Investigators and research personnel are required to complete the Collaborative Institutional Training Initiative (CITI) Human Subjects Research training course (s) prior to approval of their study: It is the position of the Rice IRB that all personnel on IRB protocols require both an appropriate basic training course ...
How long is CITI certification good for?
CITI certification is valid for 3 years. CITI will email you an automatic training reminder 60 days before your training expires and prompt you to complete a Refresher Course for recertification.
What is human research protection?
The Human Research Protection Program provides educational resources and training opportunities for those involved in human subjects’ research. Faculty can arrange for class or lab presentations to discuss ethics, the history of human subjects’ research and/or application process.
Does GCP replace IRB training?
GCP training does not replace the standard human subject research training required by the IRB. For Question 6, select the box (es) that most closely align with your research. In most cases, this will reflect the other course selected. For example:
What is CITI training?
What is CITI? The Collaborative IRB Training Initiative Program ( CITI) is a leading online training program maintained by the University of Miami. It offers curricula in human subjects research, animal research, and the responsible conduct of research.
How to merge CITI accounts?
In order to merge multiple accounts into just one CITI Program account, please send an e-mail to [email protected] and include the following information: 1 Your first and last name. 2 The name of your institution. 3 The Member ID for the account to KEEP (in other words, the account with the most up-to-date information). 4 The Member ID for the account to merge. It is IMPORTANT to make sure you have correctly identified which account you want to keep and which you want to merge.
Is RCR required for IRB?
RCR is not required by the Emory IRB and will not count towards your training requirements. For more information about what courses you need to take, please see the guidance above entitled " Step-by-Step Guidance for Selecting Required Courses and Obtaining a CITI Account ".
How long does it take to complete a CITI course?
The CITI website notes that the average learner spends about 4 ½ hours completing a basic course and about 2 hours completing a refresher course. You do not have to complete the entire course in one sitting; the CITI program is set up so that you can step away from it and start again where you left off previously.
What is RCR in research?
A University of Iowa Responsible Conduct of Research (RCR) Plan has been created under the joint sponsorship and responsibility of the Graduate College and the Office of the Vice President for Research to ensure funded investigators are formally trained in RCR. For contact information, FAQs, a list of training requirements, and to view the UI Responsible Conduct of Research Plan, please click on the RCR link above.
Do I need to take a refresher course for CITI?
CITI training is a one-time requirement at the University of Iowa and researchers are NOT required by the UI IRB to take a refresher course. However, some sponsors have the requirement that researchers complete a refresher course every few years after completing the original human subject’s research training for Group 1 – Biomedical - IRB-01 and/or Group 2 – Social/Behavioral – IRB-02. The UI IRB has added refresher courses to ensure researcher compliance with their sponsor’s requirements for taking refresher courses. These courses are also good resources to any UI researcher who would like to refresh their knowledge of human subjects research.
What is GCP training?
Effective January 1, 2017: All investigators and staff who are involved in the conduct, oversight or management of NIH funded clinical trials are required to complete training in Good Clinical Practice (GCP), consistent with principles of the International Conference on Harmonisation (ICH) E6 (R2) and refresh this training every 3 years. Information on this training requirement can be found here . Investigators will be expected to retain their own documentation of completion of this training as well as their human subjects protections training. This policy applies to all active grants and contracts, including those awarded before January 1, 2017. The University of Iowa will require GCP training to be completed by all researchers involved in the conduct, oversight, or management of NIH funded clinical trials no later than 12/31/17.
Who is included in a research team?
All members of the research team, including the principal investigator and all other individuals (faculty, staff, or student) who have contact or interactions with research subjects or with their private, identifiable information.
Can I take CITI certification from another university?
The University of Iowa cannot accept a CITI completion certificate from another institution. The training modules in the CITI on-line course are institution specific. Although there are a limited number of modules available through CITI, each institution establishes the groups of modules that meet their requirements for certification. For this reason, completion of the CITI course for another institution does not meet the requirements for certification at the UI.
What training is required to conduct human subjects research?
The "Biomedical Responsible Conduct of Research" course is appropriate for biomedical research studies.
What happens if I don't complete the training by the training expiration date?
For studies pending approval: The IRB cannot release an approval letter until all listed study personnel has their training verified. Failure to complete training can delay committee approval of the research project. The PI can decide to remove you from the submission to avoid delaying his/her study approval.
How can I check if my training is still good?
Under the Main Menu/My Courses, courses will be listed that require your attention. Or you can go under My Records to access course completion data and print completion certificates.
Where do I send my completion certificate?
There is no need to email or send in the CITI certificate if you are under the TAMU-CC CITI account (see below on how to affiliate with TAMU-CC). We can look up individual’s training records directly within CITI.
on This Page
Who Should Take Citi Training?
- Completion of human subject training should be done by all staff working on a research project (all investigators and other study personnel, including all persons who are responsible for designing and/or conducting research including consenting prospective participants, performing data analysis or reporting activities - NOTE: persons who function solely as administrative conta…
Getting Started and Registration
- Stanford users: 1. Go to the CITI websiteto create a CITI account. You will be directed to the Stanford WebLogin - your SUNet ID and Password will be your CITI username and login information. 2. Once logged in, you will be guided to your CITI Main Menu page, where you can choose courses and print completion reports. If you do not have a SUNet ID, you must request o…
General Information About Taking The Coursework
- Selecting a Learner Group There are seven Learner Groups to choose from, with each group reflecting a different research area of focus. Group descriptions are provided when you register for CITI. Choose a Learner Group that best reflects your area of research and responsibilities. Investigators and research staff: Group 7 or Group 2, depending on whether medical or nonmedi…
Timing
- Can I complete this training in several sessions? Yes, you can leave and come back to the site using your login credentials as often as necessary. All completed work is stored. How often do I have to take this course to keep my training current? Training is required every three years. You will need to access the CITI tutorial again to take the Refresher course, so make sure you record …
Transferring Training from Another Institution
- Transfer CITI training completed at another institution:
- Transfer non-CITI training completed at another institution:
Obtaining A Copy of The Completion Certificate
- You can view and print a copy of your completion certificate by logging into the CITI home page. At the "Learner's Menu" screen, if the Status heading says: 1. "Passed": click on the PRINT link under Completion Reports. This will display a table. Click “Print Completion Report” to print the certificate. 2. "Not Started - Enter" or"Incomplete - Re-enter": click on Previous Coursework Compl…
Popular Posts:
- 1. 7. where does legislation concerning employer practices come from? course hero
- 2. how much is a wilderness survival course for a week
- 3. how to show course completion on resume
- 4. who is responsible for ensuring a course qualifies for cpe credit? r156-9-304-5
- 5. what is the course id number for vsb cle indigent defense course in may 2015
- 6. how do you spell of course'
- 7. how much is it to play golf at fort benning glof course
- 8. how to join the computer course at home
- 9. what kind of pine trees are on the kapalua golf course in maui
- 10. which command will establish a new roll course hero