What determines the amino acid properties of proteins and peptides?
Mar 12, 2012 · The α-amino acids found in proteins have another important structural feature called an R group. This feature varies from amino acid to amino acid and invests these compounds with distinct chemical and physical properties. Biochemists have some understanding about these R groups’ physicochemical utility.
What are the unique chemical and structural properties of amino acids?
Sep 24, 2018 · structures and chemical properties Surface charge representation of the proteins actin (A) and HIV protease (B). Even though both proteins are chains of amino acids, they each feature distinct three-dimensional shapes with unique chemical properties, as evidenced by the unique distribution of surface charges on each molecule.
Are amino acids weak or strong acids?
pKa Table for amino acids: * First column (pKa 1) = COOH * Second column (pKa 2) = NH 3 + * Third column (pKa R) = R group H + AMINO ACIDS AS WEAK ACIDS: - Properties of amino acids in proteins and peptides are determined by the R group but also by the charges of the titratable group. Will ultimately affect protein structure.
What is the difference between amino acids and proteins?
Sep 18, 2020 · Amino acids have high melting point (200-300) o C due to ionic property. Solubility: Solubility of amino acids depends upon polarity, iso-electric point, nature of solvent (pH) and temperature. Amino acids are soluble in water and ethanol (i.e. polar solvent) and insoluble in non-polar solvent like benzene, ether etc.
Why do different amino acids have different properties?
Each of the 20 amino acids has a specific side chain, known as an R group, that is also attached to the α carbon. The R groups have a variety of shapes, sizes, charges, and reactivities. This allows amino acids to be grouped according to the chemical properties of their side chains.
What gives amino acids its unique chemical properties?
Each amino acid has unique characteristics arising from the size, shape, solubility, and ionization properties of its R group. As a result, the side chains of amino acids exert a profound effect on the structure and biological activity of proteins.Nov 4, 2021
What are the chemical properties of amino acids?
Properties of amino acids: physical and chemicalAmino acids are colorless, crystalline substance.Most amino acids are tasteless but some are sweet. ( E.g. Glycine, Alanine) and some are bitter (Eg. ... Amino acids have high melting point (200-300)oC due to ionic property.Solubility:Sep 18, 2020
How are amino acids different from each other?
Amino acids differ from each other with respect to their side chains, which are referred to as R groups. The R group for each of the amino acids will differ in structure, electrical charge, and polarity.
What part of each amino acid makes it unique?
side groupsThe side groups are what make each amino acid different from the others. Of the 20 side groups used to make proteins, there are two main groups: polar and non-polar. These names refer to the way the side groups, sometimes called "R" groups, interact with the environment.
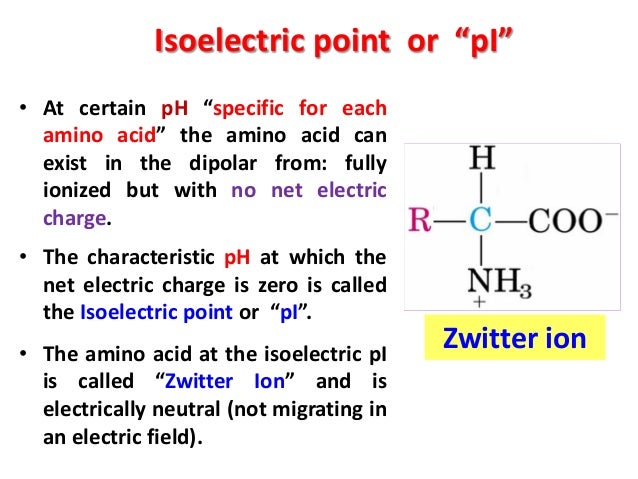
Why do amino acids have different isoelectric points?
The isoelectric point of an amino acid is the point at which the amino acid has no net electrical charge. It is an important characteristic for any amino acid, because every amino acid has at least two acid–base (titratable) groups.
Which amino acids have similar properties?
According to Grantham's distance, most similar amino acids are leucine and isoleucine and the most distant are cysteine and tryptophan.
What chemical properties of amino acids proteins make them so diverse?
Their structures, like their functions, vary greatly. They are all, however, polymers of amino acids, arranged in a linear sequence. The functions of proteins are very diverse because there are 20 different chemically distinct amino acids that form long chains, and the amino acids can be in any order.
What is the chemical structure of amino acid?
An amino acid is an organic molecule that is made up of a basic amino group (−NH2), an acidic carboxyl group (−COOH), and an organic R group (or side chain) that is unique to each amino acid. The term amino acid is short for α-amino [alpha-amino] carboxylic acid.
How do amino acids differ from one another quizlet?
How do amino acids differ from one another? The main difference among the different amino acids is in their R groups. In the induced fit model of enzyme action, the enzyme then reduces the activation energy of the reaction so reactants can become products. The enzyme is unchanged and is available to be used again.
How do the differences in amino acid sequences lead to different protein functions?
How do the differences in amino acid sequences lead to different protein functions? Different amino acids produce different proteins based on the bonds formed between them. What causes the changes in protein structure through the three or four levels of structure?
Which part of the amino acid varies between different types of amino acids?
Each amino acid shares a common set of atoms that make up the amino acid backbone. Attached to the central carbon atom (the alpha carbon) is an atom or group of atoms that varies among the amino acids, making them all different. This group is sometimes called the R group or amino acid sidechain.
How are amino acids connected?
Amino acids are connected by peptide bonds in proteins. The amino and carboxylic acid groups of any two amino acids can be covalently connected by a peptide bond, with the equivalent of the removal of a water molecule (shown in blue). The resulting amino acid chain has two ends, one with a free amino group, the N-terminus, and one with a free carboxylic acid group, the C-terminus. (B) Proteins are often made from very long polypeptide chains that typically contain hundreds of peptide bonds. Only four amino acid subunits of a protein are shown, as depicted by the squiggly lines (“spinach”) at each end.
What is the chemical group of amino acids?
carbon. For 19 of the 20 amino acids, an additional chemical group, known as an R group or side chain, is attached to the α carbon. The twentieth amino acid, glycine, has two hydrogen atoms connected to the α carbon instead of an R group and a single hydrogen atom. The unique chemical and structural properties of each amino acid are determined by the identity of the R group.
What is the order of amino acids in a protein?
The specific order of amino acids in a protein is known as its primary structure. It is this sequence that determines the three-dimensional architecture of a protein. A famous experiment that proves that all the information necessary for proper folding of a protein is contained in its primary structure is presented in the next chapter.
What is crystal structure?
An X-ray crystal structure describes the near- exact position of each atom in the molecule in three-dimensional space. The technique for obtaining such a structure involves the use of X-rays (a form of electromagnetic radiation) and a crystal of the molecule in question. A . p53 protein DNA double helix.
What is the amino acid cysteine?
The amino acid cysteine contains a sulfhydryl (-SH) group on its side chain. Sulfhydryls can be oxidized to form disulfide bonds in which two cysteine side chains, often from distant locations in the primary sequence, come together to form a sulfur-sulfur covalent bond (Figure 18). The formation of such disulfide bonds in proteins rigidifies the protein and can stabilize conformations that are otherwise not particularly favored. A pair of disulfide-bonded cysteine residues is collectively known as cystine.
What are the side chains of proteins?
The amino acid side chains we have been describing are key functional components of proteins. Many proteins have enzymatic functions, and in these cases the side chains are directly involved in the reactions the enzymes catalyze. One class of enzymes, proteases, has evolved to catalyze the breakdown of peptide bonds in proteins. Many proteases contains a “catalytic triad” in which three amino acids in a precise arrangement work together to hydrolyze peptide bonds (Figure 19). The catalytic triad involves serine, histidine, and aspartate. The enzyme uses this catalytic triad to facilitate enzyme-catalyzed peptide bond hydrolysis. Of course, this can only occur because the folded protein precisely positions each of these amino acids in three dimensions, enabling the amino acids to interact with each other and with the substrate in just the right way. We will learn more about enzyme catalysis in later chapters, but before we do that, we need to examine the folded structures of proteins and the thermodynamics that underlie their formation.
What are the most diverse and versatile macromolecules found in living systems?
Proteins are the most diverse and versatile macromolecules found in living systems. Proteins are polymeric chains of amino acid monomers connected by covalent peptide bonds. Unlike most other biological polymers, proteins fold into unique structures with distinct physical and chemical properties. As a result, cells use proteins for a broad range of structural, catalytic, regulatory, and transport functions. The folded structure of a protein is solely determined by its amino acid sequence, or primary structure. In fact, even small changes in the amino acid sequence can potentially alter a protein ’s folded structure, as we saw in the example of sickle-cell disease.
What are the functions of amino acids?
Functions of amino acids: Precursor for synthesis of proteins and polypeptides. Used for glucogenic and ketogenic degradation. For synthesis of porphyrin. For synthesis of melan in: Melanin is complex polymeric structure made up of tyrosine and also may contain tryptophan.
What is the titration curve of amino acids?
When they are in fully protonated form they can be titrated twice. Titration curve is the graph made between pH of amino acids and volume of acid or base added. It is always sigmoidal.
Is amino acid soluble in water?
Solubility of amino acids depends upon polarity, iso-electric point, nature of solvent (pH) and temperature. Amino acids are soluble in water and ethanol ( i.e. polar solvent) and insoluble in non-polar solvent like benzene, ether etc. Amino acids are insoluble at iso-electric point. Solubility depends upon pH of solvent and temperature.
How many amino acids are in proteins?
The 20 amino acids found in proteins. Both three-letter and one-letter abbreviations are listed. As shown, there are equal numbers of polar and nonpolar side chains. For their atomic structures, see Panel 3-1 (pp. 132–133).
What are the structural components of a protein?
The structural components of a protein. A protein consists of a polypeptide backbone with attached side chains. Each type of protein differs in its sequence and number of amino acids; therefore, it is the sequence of the chemically different side chains (more...)
What is a protein made of?
A protein molecule is made from a long chain of these amino acids, each linked to its neighbor through a covalent peptide bond ( Figure 3-1 ). Proteins are therefore also known as polypeptides. Each type of protein has a unique sequence of amino acids, exactly the same from one molecule to the next.
Which helix is hydrogen bonded to?
The regular conformation of the polypeptide backbone observed in the α helix and the β sheet. (A, B, and C) The α helix. The N–H of every peptide bond is hydrogen-bonded to the C=O of a neighboring peptide bond located (more...)
How many genes are in the human genome?
The result of sequencing the human genome has been surprising, because it reveals that our chromosomes contain only 30,000 to 35,000 genes. With regard to gene number, we would appear to be no more than 1.4-fold more complex than the tiny mustard weed, Arabidopsis, and less than 2-fold more complex than a nematode worm. The genome sequences also reveal that vertebrates have inherited nearly all of their protein domains from invertebrates—with only 7 percent of identified human domains being vertebrate-specific.
What type of bonds help proteins fold?
Three types of noncovalent bonds that help proteins fold. Although a single one of these bonds is quite weak, many of them often form together to create a strong bonding arrangement, as in the example shown. As in the previous figure, R is used as a general (more...)
How do proteins help maintain their structure?
To help maintain their structures, the polypeptide chains in such proteins are often stabilized by covalent cross-linkages. These linkages can either tie two amino acids in the same protein together, or connect different polypeptide chains in a multisubunit protein. The most common cross-linkages in proteins are covalent sulfur–sulfur bonds. These disulfide bonds (also called S–S bonds) form as proteins are being prepared for export from cells. As described in Chapter 12, their formation is catalyzed in the endoplasmic reticulum by an enzyme that links together two pairs of –SH groups of cysteine side chains that are adjacent in the folded protein ( Figure 3-29 ). Disulfide bonds do not change the conformation of a protein but instead act as atomic staples to reinforce its most favored conformation. For example, lysozyme —an enzyme in tears that dissolves bacterial cell walls—retains its antibacterial activity for a long time because it is stabilized by such cross-linkages.
What is the structure of an amino acid?
Amino acids are the monomers that make up proteins. Each amino acid has the same fundamental structure , which consists of a central carbon atom, also known as the alpha (α) carbon, bonded to an amino group (NH 2 ), a carboxyl group (COOH), and to a hydrogen atom.
What are the key points of amino acids?
Key Points. Each amino acid contains a central C atom, an amino group (NH2), a carboxyl group (COOH), and a specific R group. The R group determines the characteristics (size, polarity, and pH) for each type of amino acid. Peptide bonds form between the carboxyl group of one amino acid and the amino group of another through dehydration synthesis.
How do peptide bonds form?
Peptide bonds form between the carboxyl group of one amino acid and the amino group of another through dehydration synthesis. A chain of amino acids is a polypeptide.
Which amino acid is polar?
The chemical composition of the side chain determines the characteristics of the amino acid. Amino acids such as valine, methionine, and alanine are nonpolar (hydrophobic), while amino acids such as serine, threonine, and cysteine are polar (hydrophilic). The side chains of lysine and arginine are positively charged so these amino acids are also ...
How many amino acids are there in the human body?
The name “amino acid” is derived from the amino group and carboxyl-acid-group in their basic structure. There are 21 amino acids present in proteins, each with a specific R group or side chain. Ten of these are considered essential amino acids in humans because the human body cannot produce them and they must be obtained from the diet.
What is the R group in amino acids?
Every amino acid also has another atom or group of atoms bonded to the central atom known as the R group. This R group, or side chain, gives each amino acid proteins specific characteristics, including size, polarity, and pH. Amino acid structure Amino acids have a central asymmetric carbon to which an amino group, a carboxyl group, ...
How many types of amino acids are there?
Types of amino acids There are 21 common amino acids commonly found in proteins, each with a different R group (variant group) that determines its chemical nature. The 21st amino acid, not shown here, is selenocysteine, with an R group of -CH 2 -SeH.
Popular Posts:
- 1. what field of study is the best preparation for golf course superintendent
- 2. from where we can do sap perfromance engineer course in atlanta
- 3. how much does the general assembly android course cost?
- 4. how to change a course id in mblackboard
- 5. what is the easiest college course to take
- 6. how to get your pe review course paid for by someone else
- 7. how to add basic life support course to nremt
- 8. how often can you take a defensive driving course in va
- 9. what golf course is the pga at this week
- 10. where is the information stored in dna? course hero