What is the activation energy for exothermic reaction?
as an example of an exothermic reaction. Figure 12.4: The energy changes that take place during an exothermic reaction. The activation energy is the difference between the energy of the reactants and the maximum energy (i.e. the energy of the activated complex).
What is the activation energy in a reaction?
Activation energy is defined as the minimum amount of extra energy required by a reacting molecule to get converted into product. It can also be described as the minimum amount of energy needed to activate or energize molecules or atoms so that they can undergo a chemical reaction or transformation.
What factors affect activation energy in a reaction?
Determining the Activation Energy of a Reaction As the temperature increases, the molecules move faster and therefore collide more frequently. The molecules also carry more kinetic energy. Thus, the proportion of collisions that can overcome the activation energy for the reaction increases with temperature.
What is activation energy in Arrhenius equation?
The Arrhenius equation is sometimes expressed as k = Ae-E/RT where k is the rate of chemical reaction, A is a constant depending on the chemicals involved, E is the activation energy, R is the universal gas constant, and T is the temperature.
What is activation energy easy definition?
activation energy, in chemistry, the minimum amount of energy that is required to activate atoms or molecules to a condition in which they can undergo chemical transformation or physical transport.
What is activation energy examples?
This energy is called activation energy. For example, activation energy is needed to start a car engine. Turning the key causes a spark that activates the burning of gasoline in the engine. The combustion of gas won't occur without the spark of energy to begin the reaction.
What is the relationship between activation energy and reaction rate?
The activation energy of a chemical reaction is closely related to its rate. Specifically, the higher the activation energy, the slower the chemical reaction will be. This is because molecules can only complete the reaction once they have reached the top of the activation energy barrier.
What is activation energy and how do catalysts affect it?
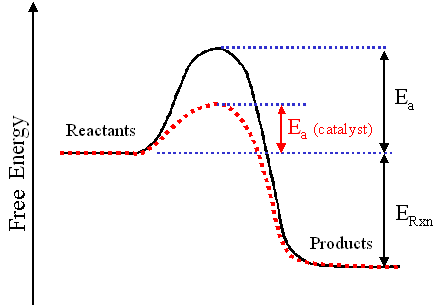
A catalyst increases the rate of reaction by decreasing the activation energy. Decreased activation energy means less energy required to start the reaction. The graph below shows the energy of a reaction both with and without a catalyst present.
What are the 4 factors that affect the rate of reaction?
The rate of a chemical reaction is influenced by many different factors, including reactant concentration, surface area, temperature, and catalysts.
What is the Arrhenius factor?
The pre-exponential factor (A) is an important component of the Arrhenius equation, which was formulated by the Swedish chemist Svante Arrhenius in 1889. The pre-exponential factor is also known as the frequency factor, and represents the frequency of collisions between reactant molecules at a standard concentration.
How do you find the activation energy of A Arrhenius graph?
0:385:32How to Use an Arrhenius Plot To Calculate Activation Energy and ...YouTubeStart of suggested clipEnd of suggested clipHere to do an arrhenius plot all you need to do is on your x-axis. You have one over yourMoreHere to do an arrhenius plot all you need to do is on your x-axis. You have one over your temperature so your inverse temperature make sure it's in kelvin. Um so the inverse temperature will be kelvin
How do you find the Arrhenius factor?
The Arrhenius equation is k = Ae^(-Ea/RT), where A is the frequency or pre-exponential factor and e^(-Ea/RT) represents the fraction of collisions that have enough energy to overcome the activation barrier (i.e., have energy greater than or equal to the activation energy Ea) at temperature T.
How do you calculate activation energy?
Activation Energy ProblemStep 1: Convert temperatures from degrees Celsius to Kelvin. T = degrees Celsius + 273.15. T1 = 3 + 273.15. ... Step 2 - Find Ea ln(k2/k1) = Ea/R x (1/T1 - 1/T2) ... Answer: The activation energy for this reaction is 4.59 x 104 J/mol or 45.9 kJ/mol.
Why activation energy is related to rate of reaction?
The activation energy of a chemical reaction is closely related to its rate. Specifically, the higher the activation energy, the slower the chemical reaction will be. This is because molecules can only complete the reaction once they have reached the top of the activation energy barrier.
What is the definition of activation energy in biology?
activation energy: The minimum energy required for a reaction to occur. catalysis: The increase in the rate of a chemical reaction by lowering its activation energy.
What is the activation energy quizlet?
activation energy. The amount of energy required to cause a chemical reaction;specifically the energy required to reach the transition state.
What is activation energy?
Updated July 17, 2019. Activation energy is the amount of energy that needs to be supplied in order for a chemical reaction to proceed. The example problem below demonstrates how to determine the activation energy of a reaction from reaction rate constants at different temperatures.
Who Discovered Activation Energy?
Swedish scientist Svante Arrhenius proposed the term "activation energy" in 1880 to define the minimum energy needed for a set of chemical reactants to interact and form products. In a diagram, activation energy is graphed as the height of an energy barrier between two minimum points of potential energy. The minimum points are the energies of the stable reactants and products.
What is the gas constant of R?
where m is the slope of the line, Ea is the activation energy, and R is the ideal gas constant of 8.314 J/mol-K. If you took temperature measurements in Celsius or Fahrenheit, remember to convert them to Kelvin before calculating 1/T and plotting the graph.
What are the minimum points of a reaction?
The minimum points are the energies of the stable reactants and products. Even exothermic reactions, such as burning a candle, require energy input. In the case of combustion, a lit match or extreme heat starts the reaction. From there, the heat evolved from the reaction supplies the energy to make it self-sustaining.
Does temperature affect reaction rate?
Keep in mind, while most reaction rates increase with temperature, there are some cases where the rate of reaction decreases with temperature. These reactions have negative activation energy. So, while you should expect activation energy to be a positive number, be aware that it's possible for it to be negative as well.
Popular Posts:
- 1. what kind madaical course are usa?
- 2. what is the full course for becker cpa
- 3. crash course world history what grade level
- 4. how to use azure course credits
- 5. how to find mindtap course key
- 6. what is course number in a transfer paper
- 7. how many credit hours in one ut course
- 8. how to c hange your handicap for an executive course
- 9. how to purchase a golf course
- 10. in space what happens over the course of 11 years?