How is coarse pearlite formed?
At what temperature does coarse pearlite form?
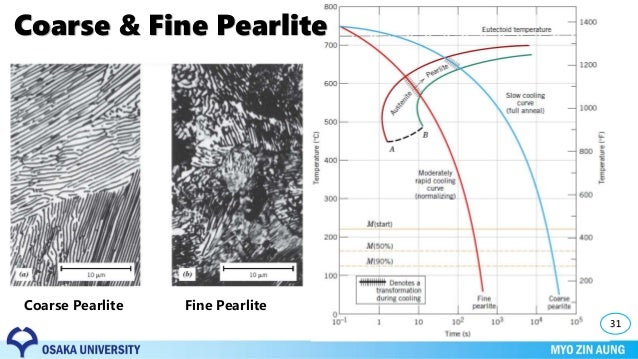
Why is pearlite coarser at higher temperature?
What's the difference between fine and coarse pearlite?
Is pearlite a BCC?
Is ferrite harder than cementite?
Which is harder pearlite or martensite?
Can pearlite become austenite?
What is pearlite structure?
What is the difference between martensite and pearlite?
What is the difference between pearlite and bainite?
Is coarse pearlite more ductile than fine pearlite?
Is pearlite hard or soft?
Pearlite can be hard and strong but is not particularly tough. It can be wear-resistant because of a strong lamellar network of ferrite and cementite. Examples of applications include cutting tools, high strength wires, knives, chisels, and nails .
What is pearlite made of?
Pearlite is a two-phased, lamellar (or layered) structure composed of alternating layers of ferrite (87.5 wt%) and cementite (12.5 wt%) that occurs in some steels and cast irons. During slow cooling of an iron-carbon alloy, pearlite forms by a eutectoid reaction as austenite cools below 723 °C (1,333 °F) (the eutectoid temperature).
How is bainite prepared?
It is prepared by more rapid cooling. Unlike pearlite, whose formation involves the diffusion of all atoms, bainite grows by a displacive transformation mechanism. The transformation of pearlite to austenite takes place at lower critical temperature of 723C.
Can eutectoid steel be pearlitic?
Eutectoid steel can in principle be transformed completely into pearlite; hypoeut ectoid steels can also be completely pearlitic if transformed at a temperature below the normal eutectoid. Pearlite can be hard and strong but is not particularly tough. It can be wear-resistant because of a strong lamellar network of ferrite and cementite. Examples of applications include cutting tools, high strength wires, knives, chisels, and nails .
What do the red dots on the graph mean?
The red dots indicate the positions of carbon atoms. Iron atoms are not shown. The nanotube is shown for size reference. Pearlite occurs at the eutectoid of the iron-carbon phase diagram (near the lower left). Pearlite is a two-phased, lamellar (or layered) structure composed of alternating layers of ferrite (87.5 wt%) and cementite (12.5 wt%) ...
2.21 Formation of Pearlite in Eutectoid Steel
Have you ever wondered why ceramics are hard and brittle while metals tend to be ductile? Why some materials conduct heat or electricity while others are insulators? Why adding just a small amount of carbon to iron results in an alloy that is so much stronger than the base metal? In this course, you will learn how a material’s properties are determined by the microstructure of the material, which is in turn determined by composition and the processing that the material has undergone.
SEE MORE
If thermodynamics, which we covered in the previous module, tells us how a material wants to change, then kinetics tells us how and how quickly that transformation occurs. This module starts by explaining the driving force for phase transformations. We will cover the nucleation and growth of precipitates, solidification, and sintering.
What temperature does pearlite form?
In an Fe–C alloy of eutectoid composition, pearlite is the only product formed from the eutectoid temperature, 727 °C, down to about 600 °C. Small amounts of bainite appear at 600 °C. The proportion of bainite increases with decreasing temperature, and at 500 °C the amounts of pearlite and bainite are about equal.
What is pearlite made of?
Pearlite is the product of the decomposition of austenite by a eutectoid reaction and comprises a lamellar arrangement of ferrite and cementite. The pearlite reaction provides an excellent example of the historical development of physical metallurgy and the importance of the interaction of experimental observations and the development of quantitative models. It is interesting to note that in the classical work A History of Metallography, Smith (1960) refers to Sorby’s presentation of the first images of pearlite at the British Association meeting in September 1864 ( Sorby, 1864 ). Smith comments that the images got little response or interest and speculates that this may have been because they lacked a theoretical interpretation at the time. A copy of one of Sorby’s original images is reproduced in Fig. 8.1, in which the characteristic lamellar morphology of the two constituent phases, ferrite and cementite, of pearlite is evident.
What is the mechanism of brittle cracking of pearlite?
The mechanism of brittle cracking of pearlite in eutectoid steels has been investigated by many researchers (Alexander & Bernstein, 1989; Barnby & Johnson, 1969; Kavishe & Baker, 1986; Lewandowski & Thompson, 1987 ). However, in our work in ferrite steel, only in rare cases does the pearlite colony act as a second-phase particle to initiate the cleavage microcracking. A broken pearlite colony was observed as the nucleus of cleavage microcracking in the weld metal of a ferrite/pearlite C-Mn steel fractured by Charpy V test at − 60 °C. In Figure 3.30, two matching sides of a fracture surface show two leaves of the broken pearlite colony. The width of the pearlite colony is 10 μm in crack propagation direction, which is indicated by the river-pattern cleavage strips stemming from the broken pearlite.
What happens to austenite when it is cooled?
Once the steel is cooled below the eutectoid temperature the remaining austenite, now of the eutectoid carbon content, transforms to pearlite, which consists of alternating layers of cementite and ferrite. The amount of pearlite in the structure increases with increasing carbon content.
What is the carbon content of hypoeutectoid steel?
A hypoeutectoid steel containing as little as 0.35% carbon can be wholly converted to pearlite. Since the work of Sorby, major contributions to the understanding of pearlite nucleation and growth have been made by Mehl and Hagel (1956) and by Hillert (1962).
What is pearlite made of?
In metallurgy, pearlite is a layered metallic structure of two-phases, which compose of alternating layers of ferrite (87.5 wt%) and cementite (12.5 wt%) that occurs in some steels and cast irons. It is named for its resemblance to mother of pearl.
What are the mechanical properties of pearlite?
Its mechanical properties are a function of its microstructure, which depends upon how it is mixed with ferrite. Pearlite . In metallurgy, pearlite is a layered metallic structure of two-phases, which compose of alternating layers of ferrite (87.5 wt%) and cementite (12.5 wt%) that occurs in some steels and cast irons.
What are the two phases of metastable?
The metastable phases are: 1 Pearlite . In metallurgy, pearlite is a layered metallic structure of two-phases, which compose of alternating layers of ferrite (87.5 wt%) and cementite (12.5 wt%) that occurs in some steels and cast irons. It is named for its resemblance to mother of pearl. 2 Martensite . Martensite is a very hard metastable structure with a body-centered tetragonal (BCT) crystal structure. Martensite is formed in steels when the cooling rate from austenite is at such a high rate that carbon atoms do not have time to diffuse out of the crystal structure in large enough quantities to form cementite (Fe 3 C). 3 Bainite. Bainite is a plate-like microstructure that forms in steels from austenite when cooling rates are not rapid#N#enough to produce martensite but are still fast enough so that carbon does not have enough time to diffuse to form pearlite. Bainitic steels are generally stronger and harder than pearlitic steels; yet they exhibit a desirable combination of strength and ductility.
What is the temperature of ferrite?
Ferrite. Ferrite or α-ferrite is a body-centered cubic structure phase of iron which exists below temperatures of 912°C for low concentrations of carbon in iron. α-ferrite can only dissolve up to 0.02 percent of carbon at 727°C. This is because of the configuration of the iron lattice which forms a BCC crystal structure.
How is martensite formed?
Martensite is formed in steels when the cooling rate from austenite is at such a high rate that carbon atoms do not have time to diffuse out of the crystal structure in large enough quantities to form cementite ( Fe 3 C).
What is the most common type of stainless steel?
Austenite is present in the most commonly used type of stainless steel, which are very well known for their corrosion resistance. Graphite . Adding a small amount of non-metallic carbon to iron trades its great ductility for the greater strength. Cementite .
Is pearlite a two phase structure?
Pearlite is a two-phase structure, consisting of alternate plates of ferrite and cementite. The active nucleus is defined as the first one of ferrite, or cementite, to form with a lattice orientation with austenite, which will later be found in the transformed products. Hillert has described a series of experiments that determined relative crystallographic orientations, and the results indicate that pearlite in steel may be nucleated by either ferrite, or cementite.
What is the composition of pearlite?
Pearlite composition is a function of the relative width of the ferrite and cementite plates. Pearlite can lower its carbon content to below eutectoid (0.77% C) levels through increased ferrite width, and also can increase its carbon level through reduced ferrite width.
When was pearlite discovered?
When austenite in iron-carbon alloys is transformed isothermally below the eutectoid temperature at small undercooling, it undergoes eutectoid transformation to produce a unique micro- structure termed “pearlite”, which was discovered by Sorby in 1864.
How does austenite turn into pearlite?
The transformation of austenite to pearlite occurs by nucleation and growth. Nucleation mostly occurs heterogeneously. If the austenite is homogeneous, then the nucleation of pearlite occurs almost exclusively at the grain boundaries of austenite.
What is the active nucleus of pearlite?
The active nucleus is defined as the first one of ferrite, or cementite, to form with a lattice orientation with austenite, which will later be found in the transformed products.
Is austenite an incoherent interface?
Smith has proposed that the moving pearlite interface in contact with austenite is an incoherent high energy interface, growing into a austenite grain (γ 1 Fig. 3.25) with which the pearlitic ferrite and cementite has no orientation relationship, which has been confirmed by electron microscopy.
Why is the dependence of S0 on temperature important?
The dependence of S0 on temperature is very important, not only because it measures an important characteristic of the pearlitic transformation, but also helps to determine its growth rate. The diffusion of carbon in austenite can be rate controlling. The diffusion distance is half the interlamellar spacing, S 0. As the spacing increases, the diffusion distance increases, and thus, the concentration gradient decreases, which results in a decrease in growth rate.
Popular Posts:
- 1. how to say but of course in french
- 2. how many hours is each ap course
- 3. protrain med term class how to get result of course
- 4. what is three quarters of a course length
- 5. how hard is vcu's online mass communications course
- 6. how do i buy an extension for a byu course
- 7. what is a project management office (pmo)? . course hero
- 8. character does, says, and thinks and how the character changes over the course of the novel.
- 9. how to sell a course using click funnels
- 10. how to offer online course in wordpress